Water
Investigate the geometry of water and hydrogen bonding between molecules.
Task
Create one molecule of water, run energy minimization. Check the bond length and angle between them.
Add another molecule. Run force field energy minimization. Check the bond length on both molecules, and angles between bonds. Check how the molecules are oriented to each other. Calculate energy of interaction, pay attention to the role of hydrogen bonds. Measure deformation of bonds.
Solution
| Force field | Bond length (Å) | Angle (°) |
|---|---|---|
| GAFF | 1.10 | 102.8 |
| Ghemical | 0.95 | 109.5 |
| MMFF94(s) | 0.97 | 104.0 |
| UFF | 0.96 | 104.5 |
According to Wikipedia the experimental O-H bond length is 95.84nm and the interior H-H angle is 104.5°, so both MMFF94 and UFF perform reasonably.
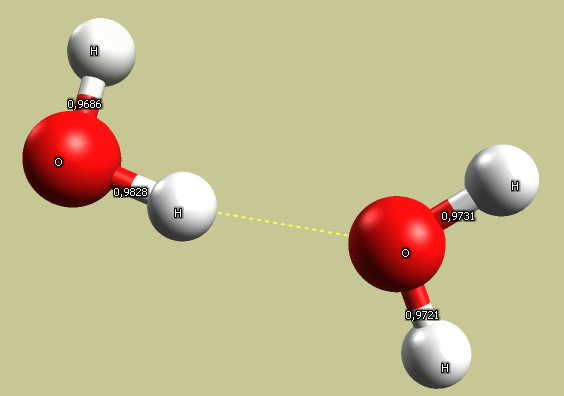
Note that there is a significant electrostatic interaction between the positively charged hydrogen atom
and the oxygen on neighboring molecules.
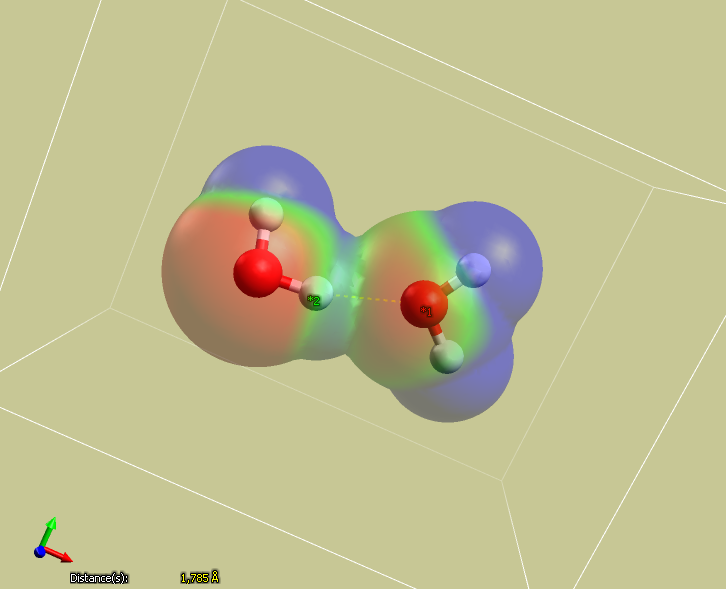
Issues
Note that only the MMFF94 and MMFF94s force fields include hydrogen bonding terms, so two water molecules may not attract each other using other methods.

Comments