Markovnikov’s Rules
Investigate the stability of reactions using Markovnikov’s rule.
Task
Build a propene molecule and optimize its geometry. Compare the relative energy of bromine-hydrogen substitution in either 1°-carbon or 2°-carbon of the carbon-carbon double bond. Which of these two bromopropane compounds will be the major product of a propene bromination reaction? 
Solution
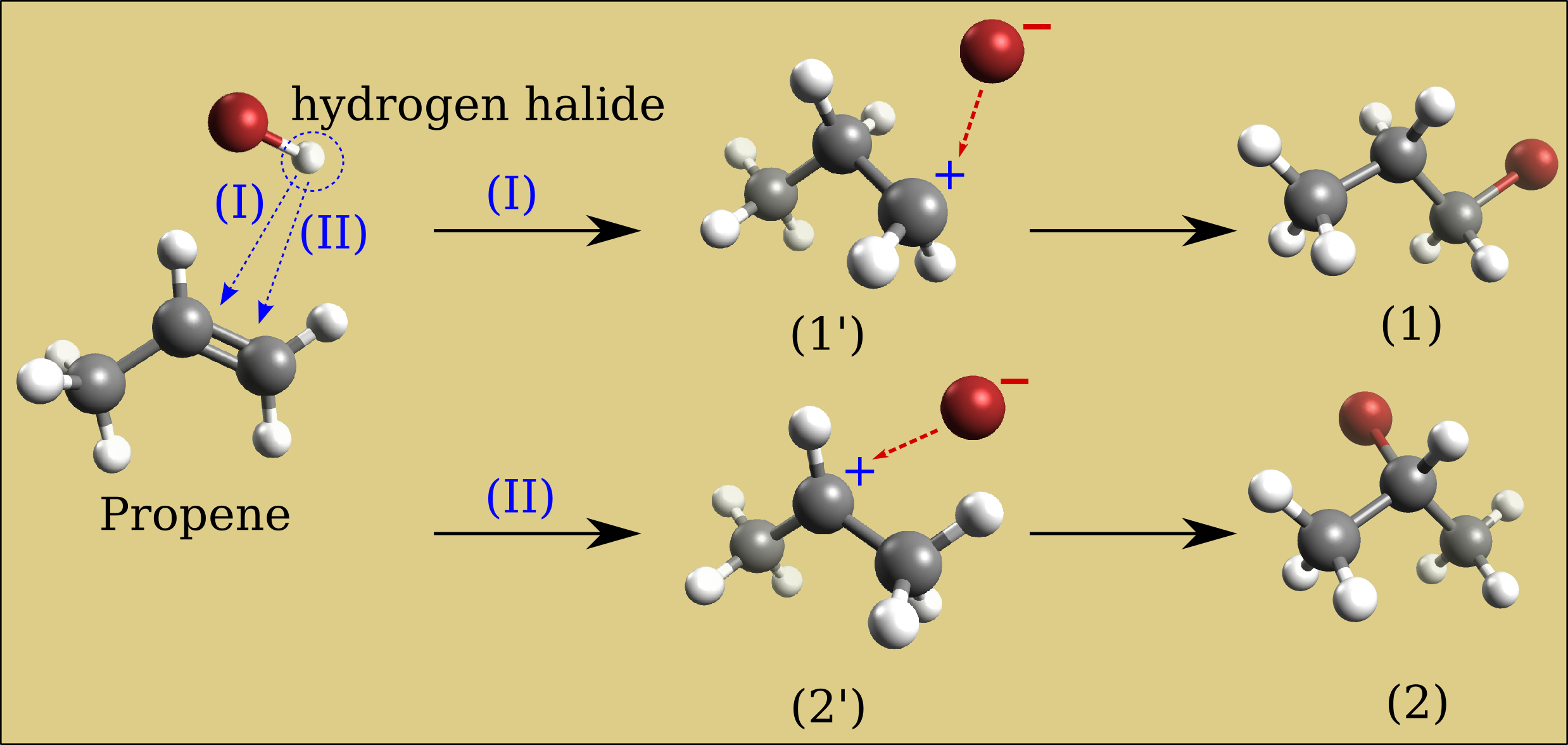
Results of relative energies expressed in kJ/mol (need confirmation in literature)
| Force field | Intermediate primary carbocation (1’) | Intermediate secondary carbocation (2’) | 1-bromopropane (1) | 2-bromopropane (2) |
|---|---|---|---|---|
| Ghemical | − 2.24 | − 2.06 | − 2.39 | − 1.87 |
| MMFF9 | − 9.88 | − 3.84 | − 8.96 | 2.70 |
| UFF | 4.05 | 3.63 | 4.15 | 4.86 |
UFF data is considered in this case, the secondary carbocation intermediate requires less energy (2’) than the primary carbocation intermediate (1’) (resp. 3.6 vs 4 kJ/mol). So the compound (2) will be the major form. This is called the Markovnikov’s rule: “the major product of the addition of HX (where X is some atom more electronegative than H, the bromine in our case) to an alkene (here the propene) has the hydrogen atom in the less substituted position and X in the more substituted position”. Mechanisms which avoid the carbocation intermediate such as the presence of dialkyl peroxides will reverse the reaction result and the compound (1) became the major product (Anti-Markovnikov rule).

Comments