Organic Hybridization
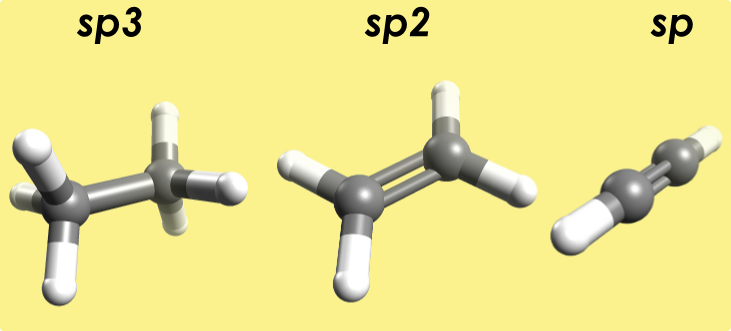
Illustrate the geometry and bond lengths in \(\ce{sp^3}\), \(\ce{sp^2}\) and \(\ce{sp}\) hybridized carbons.
Task
Build molecule and minimize geometry. Use the Measurement tool to examine bond lengths and bond angles.
Solution
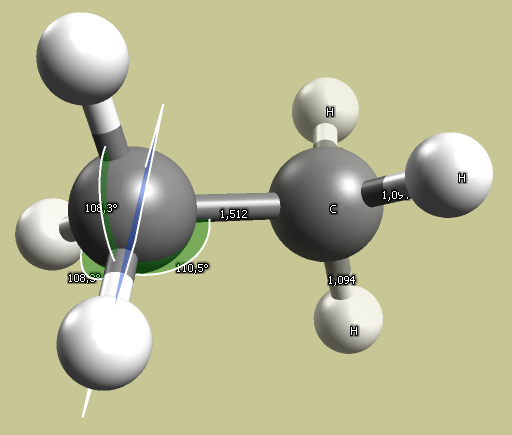
 Results using the MMFF94 forcefield (default):
Results using the MMFF94 forcefield (default):
- \(\ce{sp^3}\) - ethane bond angle: 110°; C-C bond length 1.5 A
- \(\ce{sp^2}\) - ethylene bond angle: 121°; C-C bond length: 1.336 A
- \(\ce{sp}\) - acetylene bond angle: 180°; C-C bond length: 1.200 A

Comments